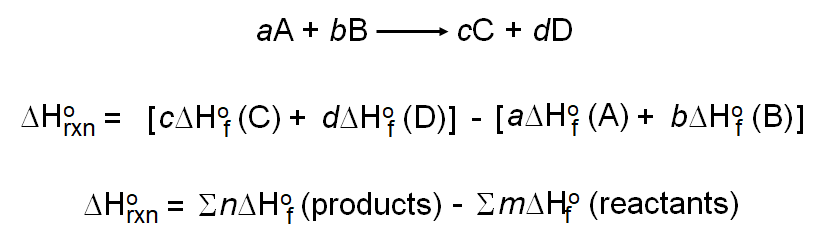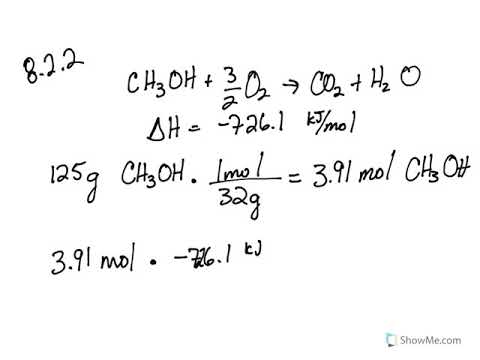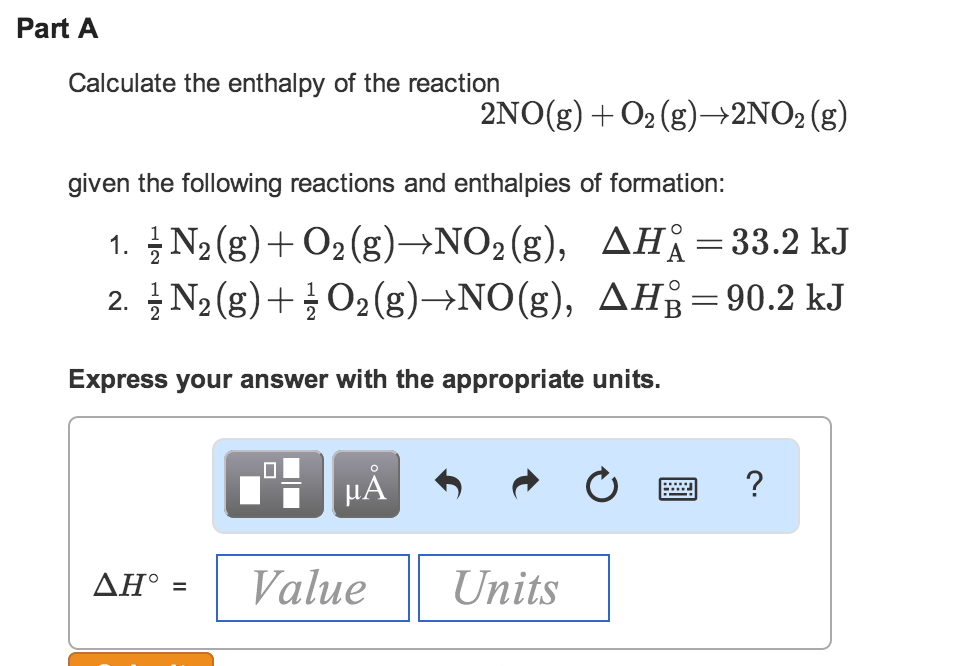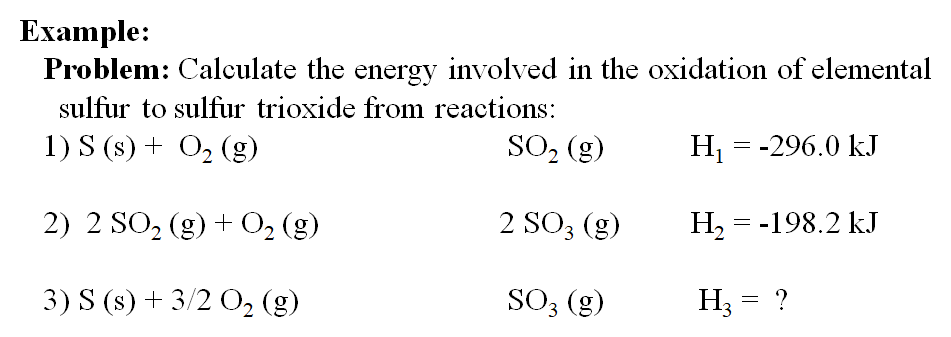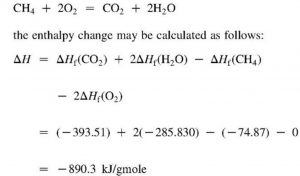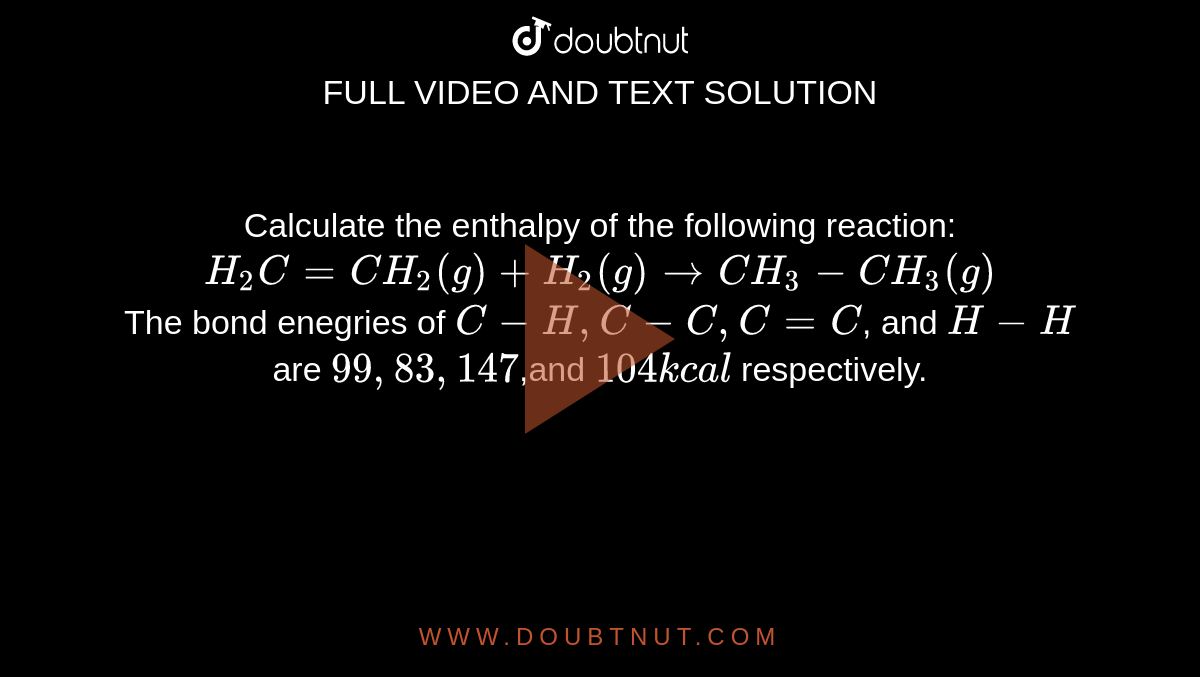
Calculate the enthalpy of the following reaction: H(2)C =CH(2)(g) +H(2)(g) rarr CH(3)-CH(3)(g) The bond enegries of C-H, C-C,C=C, and H-H are 99,83,147,and 104kcal respectively.
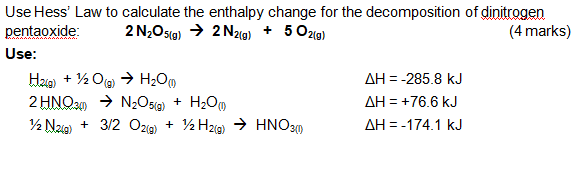
Use Hess' Law to calculate the enthalpy change for the decomposition of dinitrogen pentaoxide? | Socratic

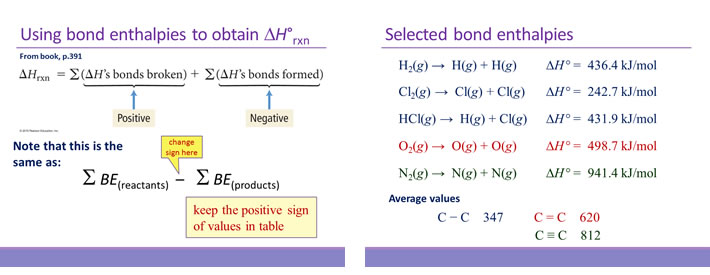
Calculate the Standard Enthalpy of the Reaction,From the Following δH° Values - Chemistry | Shaalaa.com

SOLVED: Question 3 Calculate the Enthalpy of the Photosynthesis Reaction 0/1 points Balance the reaction shown below that occurs during photosynthesis and use bond energies to estimate the enthalpy change for the

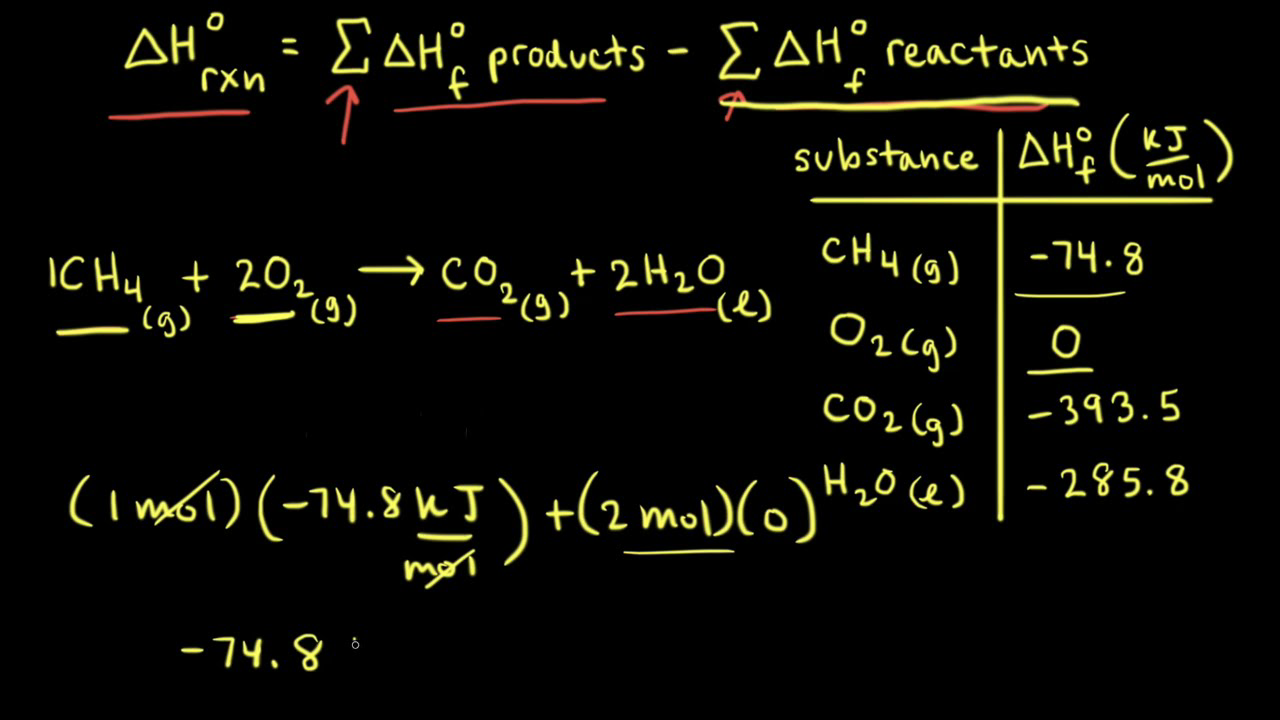
thermodynamics - Calculating Enthalpy of formation versus Calculating Enthalpy of a reaction not occurring at standard conditions - Chemistry Stack Exchange